What is the difference between alkaline and salt batteries?
Very often on store shelves there are inexpensive batteries side by side that seem to be exactly the same, but much more expensive. What is this connected with? The thing is that batteries can be saline or alkaline. Let's try to figure out how they are similar and how they differ - except for the price.
The content of the article
Salt batteries
The first prototype appeared thanks to the “flight of thought” of the Italian-born inventor Alessandro Volta back in 1800. This was the prototype of the modern salt battery. He simply took and combined several zinc and silver disks with cardboard into one, which he soaked in a salt solution. And only then scientists all over the world improved this technology and the appearance of the device.
Twenty years later, British scientist John Daniel presented a product that used zinc and copper sulfate as an electrolyte. The power of this product was 1.1 volts, however, if used in devices that do not require much electricity, their charge would be enough for a hundred years of operation.
Features of composition and design
For a long period, this battery was ahead of others in demand; this was not prevented even by the fact that the batteries have practically not changed since their creation, either externally or internally.Perfectly combining high-quality characteristics and inexpensive prices, they confidently led in sales.
The interior is very simple. The basis of the battery is the anode, which is presented in the form of powdered zinc. The active part of the battery is impregnated with manganese dioxide. Anti-corrosion elements and, naturally, an electrolyte, the role of which is played by ammonium chloride, were added to the cathode made of zinc. In fact, it was ammonium chloride that gave the battery its name, since the electrolyte in it is nothing more than salt.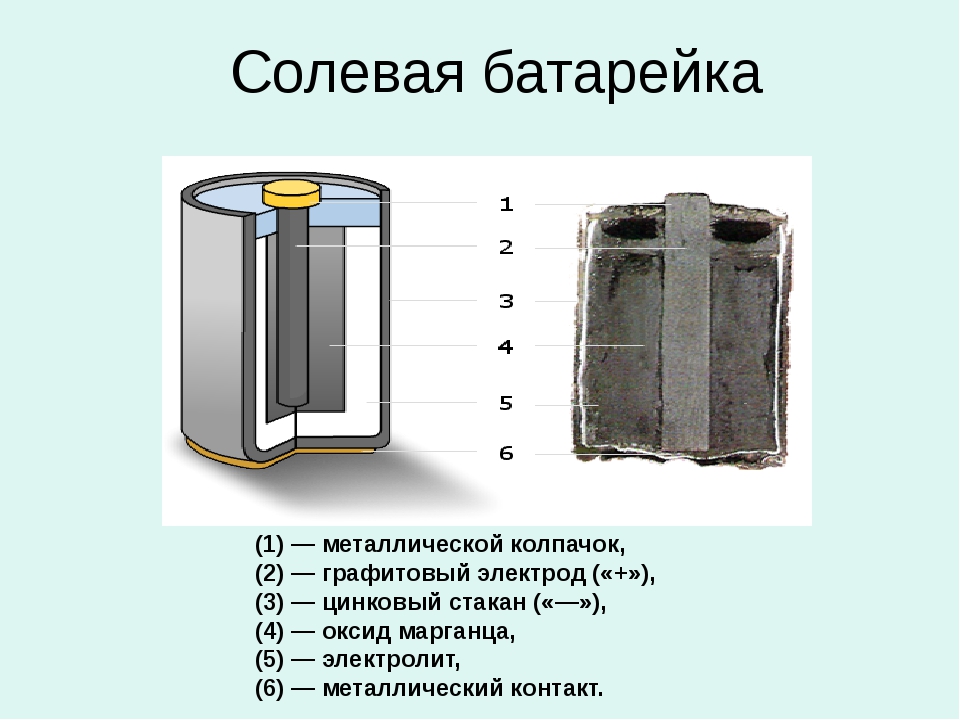
The electrodes of the cell are separated by spacers; they separate the reagent, preventing it from coming into contact, but at the same time do not prevent the penetration of the electrolyte. As a result, a reaction begins to occur inside, which is the cause of the occurrence of electric current. It flows to the nutrient elements installed inside, and through them to the electrodes, continuing its movement to the device in which the battery is placed.
Types and dimensions
In the world of electronics there are a huge number of different types of salt batteries. All these types and sizes have long acquired their own designation. The fact is that it was decided that it would be much easier to distinguish them if they were marked with letters and numbers. This solution was born in the bowels of the International Electrotechnical Commission, but in addition, similar classifications are available in our domestic GOST, TU, as well as in the imported ANSI/NEDA.
We have all long been accustomed to two types of batteries, which are easily different even in appearance. These are finger and little finger batteries. In accordance with the classification, they are assigned the designations AA and AAA, respectively. Both have a voltage of 1.5 volts.The shape of the batteries looks like an elongated cylinder.
But besides these two, there are three more varieties in stores. It is very common to see batteries classified as C or LR 14. They look like a small barrel.
Larger batteries (also in the form of a barrel, but larger) were once produced specifically for flashlights. They were marked D or LR 20, but in addition to being used in flashlights, they fit perfectly into tape recorders.
During the Soviet Union, the production of R10 batteries was launched. They found their application in various measuring instruments and... in toys.
If you look closely at the cylindrical batteries, you will notice a protrusion at one end; the plus of the batteries is located in this place. There are no protrusions at the other end, and since there are none, this means it is a minus. And in a rectangular shaped battery 6 F22, or as it is popularly called, crown, there are two protrusions on the upper part. Both plus and minus are placed in one place.
Advantages and disadvantages
If we talk about the good and the bad, we must admit that the advantages of salt batteries are lightness and low cost. These are their main trump cards. If you don't exploit them mercilessly, and sometimes give them a break, then they will last a little longer. Even if they sit down, they can be briefly revived by shaking them thoroughly, or by hitting them with your hand. With such shocking actions we will force the crumpled electrolyte to flatten out.
But there will be a little more “sad” in these batteries:
- they do not last long enough (three years and no more);
- even if the battery is not used, it will discharge itself;
- the electrolyte tends to dry out over time;
- under conditions of frequent temperature changes, the battery operates very unstable;
- This battery has some problems related to tightness, hence all kinds of leaks are possible. As a rule, this happens if the battery is not used for a long time - this causes the case to oxidize, which will inevitably cause damage to the equipment in which it is installed;
- and the main disadvantage is low energy consumption.
Alkaline batteries
Alkaline batteries appeared later. Only at the beginning of the twentieth century they were developed by the Americans Waldemar Jungner and Thomas Edison, but it took a long time for them to become popular. They are called alkaline because this is what the word “alkali” sounds like in English.
Design Features
Since alkaline batteries were invented later than salt batteries, they went on sale later. The first company to launch their production was Duracell, known to many. It still maintains a leading position in this area. Just remember their rabbit with a battery. This particular “rabbit” battery belongs to the alkaline family.
The basis of this battery, as in the case of a salt battery, is the anode - which has the form of a powder mass, which is impregnated with electrolyte. This galvanic cell uses manganese dioxide, which acts as a cathode. The electrolyte in the battery is alkali.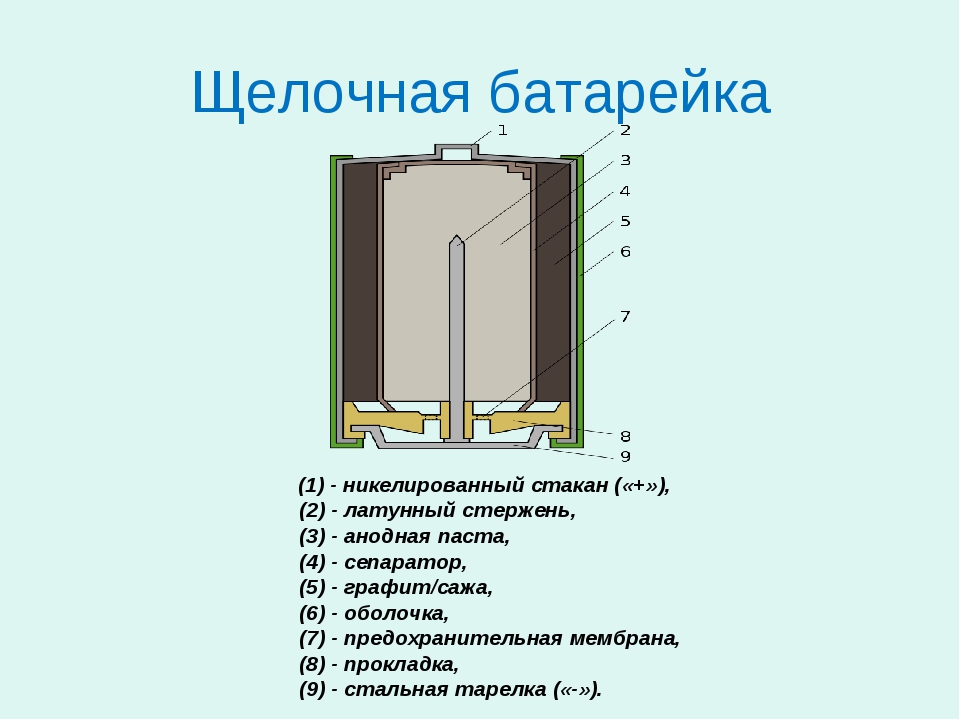
Fundamentally, the operation of an alkaline battery is no different from a salt battery, but its output is higher. A reducing agent made from zinc reduces the risk of the metal losing its activity if a strong current discharge occurs. In addition, the powdered zinc electrode allows you to increase the percentage of active substance when compared with salt batteries. And thanks to the electrolyte used - alkali, the capacity increases, which is ten times higher than that of salt analogues.
Advantages and disadvantages
Positive traits:
- high energy intensity;
- shows excellent performance results at medium loads;
- very slow self-discharge;
- does not lose performance at low temperatures;
- well sealed;
- has a long operational period - from seven to ten years.
Negative qualities:
- considerable weight;
- high price;
- If the electrolyte is discharged, the battery immediately becomes unusable.
What distinguishes alkaline from salt batteries?
The most common salt batteries are zinc ones. Salt was used as the electrolyte in this galvanic cell.
Reference. In terms of operating efficiency, alkaline batteries are seven times ahead of salt “competitors”.
In alkaline batteries, salt as an electrolyte was replaced by alkali. This made their performance better than their salt counterpart. During production, they abandoned the zinc body and decided to use the same metal, but in powder form. Therefore, when alkali interacted with the anode and cathode, much more energy began to be released.
Zinc-based salt batteries are capable of operating in a temperature range from minus twenty to plus seventy degrees. They can be used in a wide variety of devices. The operational life is two or three years.
Reference. On average, the power of a salt battery is one and a half volts.
Alkaline batteries have a longer service life. Their preservation without loss of beneficial properties reaches ten years.The use of alkali as an electrolyte allows them to maintain their working properties even at low temperatures. Their dimensions coincide with salt batteries.
Until recently, alkaline power supplies did not have the ability to recharge, but recent developments have made it possible to obtain it. Now they can be used repeatedly and can retain their charge for a very long time. This made them more environmentally friendly and gave them an added advantage.
This battery most fully meets the ever-growing needs of the market, whose power demands are constantly growing.
How to distinguish them
If you take apart a salt and alkaline battery, the differences between them will immediately catch your eye, but who will allow you to do this in a store? But how then can one not make a mistake in choosing if outwardly they are no different?
There is a way out. First of all, the difference will be the cost of the battery. Since alkaline batteries are more expensive to produce, they cost much more. In addition, on the body of the alkaline battery there is the inscription ALKALINE, which in translation means alkali.





