Difference between salt and alkaline batteries
Many household appliances require a constant supply of power to function properly. Batteries are often used for this. On sale you can find a wide variety of options for galvanic cells. But alkaline and saline ones are especially popular.
Both options are widely used in everyday life. However, few people know that the difference between the elements is not only in the name.
The content of the article
Features of salt batteries
Salt batteries are the simplest devices in which electric current is generated through a chemical reaction.
Reference. The second name for salt batteries is carbon or carbon-zinc.
This battery has low energy intensity, which explains its low cost. Therefore, the salt version is suitable for low-power devices, for example, a TV remote control or a watch. In devices with a high load or subject to frequent voltage surges, it is worth using other batteries.
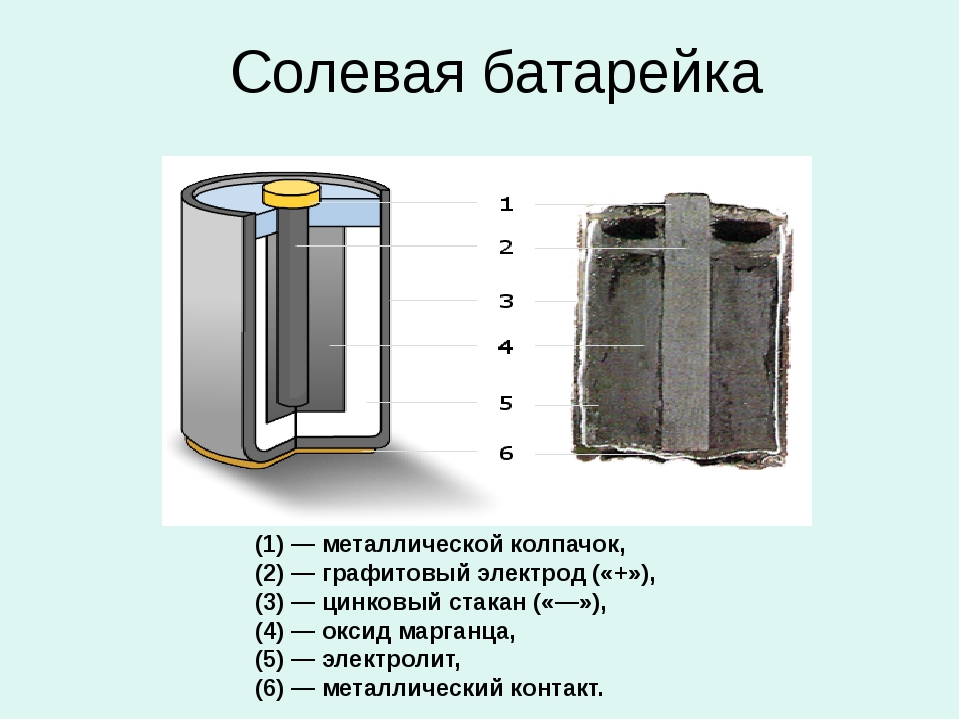
The product has a fairly simple design, which includes:
- Cathode. This is a battery housing made of zinc. Minus battery.
- Anode. Is a plus. It is made by pressing and additionally impregnated with electrolyte.
- Electrolyte. It is a substance consisting of zinc chloride or ammonium chloride. To give it a thicker consistency, a small amount of starch is added.
- Carbon conductor. Located in the center of the battery. The outer part of the current conductor is coated with a paraffin composition.
- Gas chamber. It is located in the upper part and is designed to collect gas emissions formed during a chemical reaction.
- Pad. Located below. Makes the device airtight.
The positive aspects of salt batteries include not only their low cost. Also advantages are:
- simplicity of design;
- ease of use;
- Suitable for most household appliances.
Negative features include:
- high sensitivity to temperature changes;
- short period of use and storage;
- inability to recharge;
- There is a risk of saline leakage.
What are alkaline batteries
The first alkaline battery was invented by two American scientists: Waldemar Junger and Thomas Edison. This happened at the very beginning of the twentieth century. But they became popular only years later.
Reference. Alkaline - in English means “lye”. Therefore, the second name for alkaline batteries is alkaline.
The design is not much different from salt batteries. The main difference is the location of the main parts. It also includes an anode and a cathode. The role of the anode is performed by powdered zinc, which is impregnated with potassium hydroxide. The cathode is manganese dioxide with the addition of soot or graphite. Between them there is a separator, which is also additionally impregnated with electrolyte.
The cathode and anode are enclosed in a special housing that prevents short circuits inside the device.
The chamber here is smaller, since a small amount of gases is released during the chemical reaction.
The design also includes a safety membrane, which is necessary to prevent a possible battery explosion. If the pressure increases, the membrane will rupture. Due to this, the device will be depressurized and the electrolyte will leak out.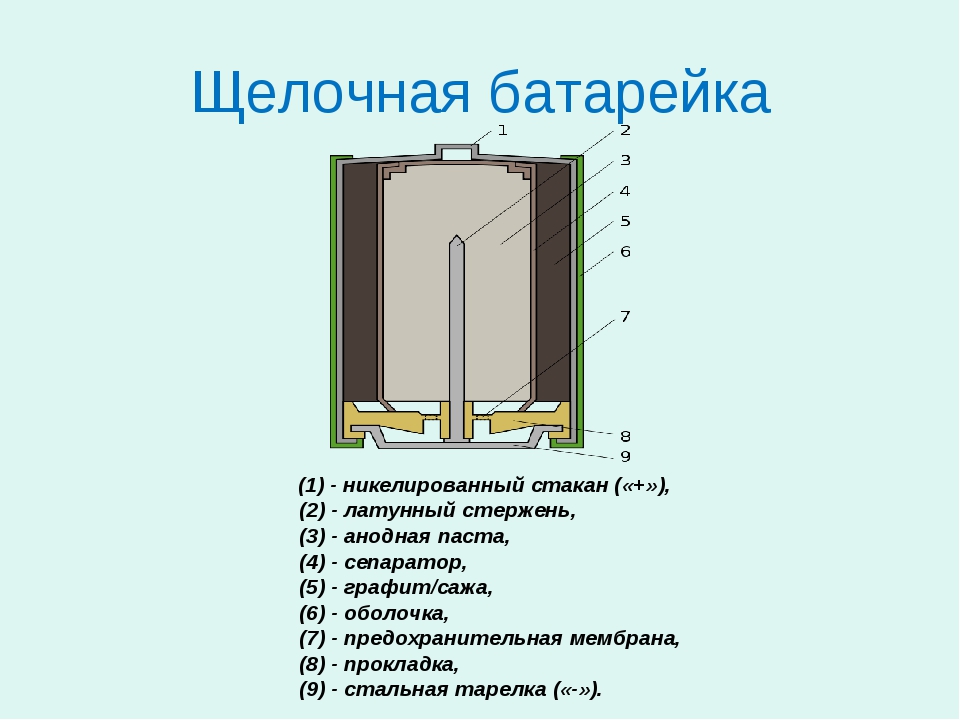
The advantages include:
- large volume;
- long service life;
- no self-discharge;
- reliable sealing of the case - so the batteries can not be removed from the device that you are not using for a long time;
- the design includes a valve that prevents spontaneous explosion;
- high specific capacity at light loads;
- works at any temperature.
The disadvantages are:
- high price;
- quite heavy weight;
- If the battery fails, it will be impossible to restore it.
Due to their large charge capacity, alkaline batteries are widely used in various devices: powerful flashlights, cameras, various remote controls, audio players and others.
There are a variety of battery options available for sale. For convenience, they have all been labeled by the International Electrotechnical Commission:
- "Pills", "buttons" or "coins". They are small round-shaped products. They are usually used in wristwatches, alarm key fobs, and kitchen scales.
- “Little finger” or micro-finger. They are designated by the combination AAA. Made in the form of a small cylinder weighing up to 15 g.Despite their miniature size, they boast high performance. Therefore, AAA batteries are widely used in a variety of children's toys.
- "Finger" They are larger in size than the “little finger” ones. Designated as AA. Also widely used in various household appliances.
- "Crown". They have a rectangular shape, two sockets and a plug. Mainly used in radio devices.

What is the difference between alkaline batteries and salt batteries?
A comparison table will help you better understand the difference between them.
| Parameter | Salt | Alkaline |
| Terms of Use. | Cannot withstand power surges and low temperatures. | They can easily withstand changes and jumps. |
| Shelf life. | 3 years. | 5 years. |
| Scope of application. | Designed for equipment with low consumption levels. | Can be used with powerful devices. |
Choosing the right battery depends on what device you are going to use it with. This must be taken into account when purchasing batteries. The instruction manual will help you make the right choice.





