Can alkaline batteries be charged?
Flashlights, children's toys, kitchen scales, electronic watches - these and many other household appliances require a miniature power source, which is a battery, to operate.
All batteries have a fairly simple design - a housing, an electrolyte and two electrodes. However, regardless of what types of them are used, sooner or later they begin to shrink. Since this can happen at the most inopportune moment, many people wonder whether it is possible to recharge an alkaline battery.
The content of the article
Design features and operating principle of an alkaline battery
Choosing the right battery depends on the device in which you will use it. One of the most popular options is alkaline or, as they are also called, alkaline batteries. They fit most appliances, last a long time, and are affordable.
Outwardly, they do not differ from other varieties, for example, salt current sources and represent a “barrel” of different diameters. But there is one design difference between salt and alkaline batteries: alkaline ones have an “inverted” design.
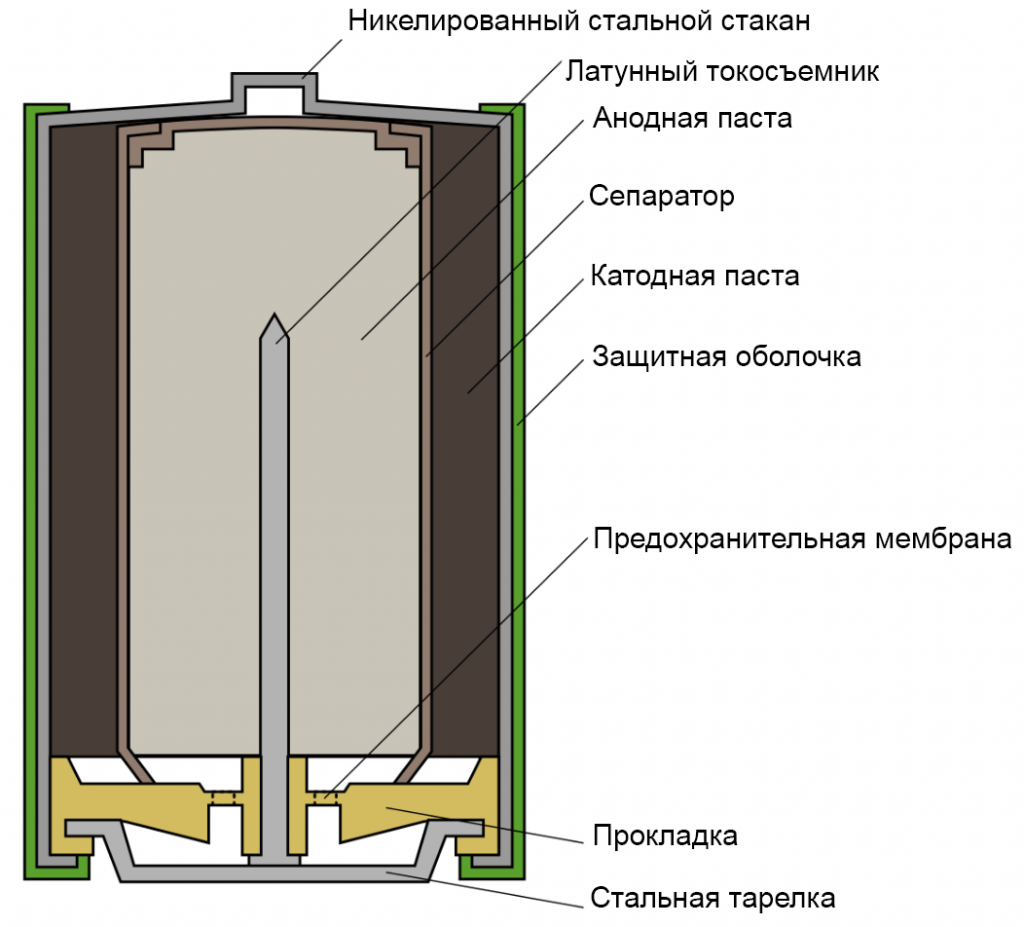
Inside the device there is zinc in powder form. Therefore, the zinc cup, which is used in salt models, is replaced here with a nickel-plated body.It not only holds the zinc powder, but also acts as a positive contact.
During operation, the positive electrode is adjacent to the walls of the metal housing. In addition, the product design allows for more manganese dioxide to be contained than is used in salt power supplies. Therefore, they have a longer service life.
Inside the housing there is a separator impregnated with electrolyte. More often it is made from hydrocellulose film.
In the central part of the product there is a cathode current conductor. An anodic substance, the role of which is played by zinc powder, is placed between it and the separator. The main thing is that the powder is impregnated with a special thickener.
Reference. Alkaline electrolyte significantly increases the life of batteries - during operation it is consumed more economically than in salt batteries.
The principle of operation of the device was first described by the Italian physicist Volta in 1782. He made a galvanic cell in which a zinc anode and a copper cathode were placed in an acidic environment. As a result of the chemical reaction, an electric current was formed.
The advantages of the device include:
- long shelf life;
- the specific capacity of alkaline current sources is twice as high as that of salt models;
- can be used in equipment with any energy protection indicator;
- not susceptible to temperature fluctuations.
Is it possible to charge an alkaline battery at home?
Sooner or later, we all encounter the fact that an alkaline power source begins to run out. During this time, our remote controls, flashlights and other devices lose functionality.But until the product is completely out of order, you can try to revive it a little by recharging it a little.
What not to do:
- do not damage the body of the power source;
- do not disassemble the battery;
- No need to bite, knock or try to cut the element capsule.
However, manufacturers urgently It is not recommended to recharge the product. After all, the outcome of such manipulations can be unpredictable. For example, the device casing may become depressurized, which will lead to electrolyte leakage.
Therefore, manufacturers took our wishes into account and began to produce models that can be charged several times.
Rechargeable alkaline batteries
There is only one option for alkaline batteries that can be charged several times - these are rechargeable devices. You can distinguish them from other devices by the corresponding marchAndleveling on the product body - Rechargeable Battery.
A new type of battery appeared in the 80s of the twentieth century. Unlike conventional batteries, such devices do not need to be pre-charged, since they can be immediately installed in the equipment. After the battery has used up its reserve, you can place it in a special device and charge it again.
Such devices are a transitional option between conventional batteries and standard batteries.
Reference. Alkaline batteries have a voltage of 1.5 volts. They are able to maintain this charge for a long time. Their main advantage is safety. And if you do not charge the product, maintaining a 25% charge, you can use the device for several years.
If you believe the manufacturers’ words, you can “recharge” a half-dead battery in the charger about 50 times.You can recharge a completely dead device 20 times.

Such power supplies have standard sizes: AA, AAA, D and others. Therefore, they can be used in all household appliances.
Despite the high cost, rechargeable batteries have found a field of application and are in stable demand.
To extend the life of batteries, try not to use them in extreme cold: this will cause them to discharge faster. In addition, before purchasing, pay attention to the release date: all current sources have a tendency to self-discharge.
Experts do not recommend installing different models, for example, alkaline and saline, in one device. This shortens the service life of the products. And if you need “long-lasting” batteries, we recommend purchasing rechargeable batteries.
At least they described the structural difference between regular batteries and rechargeable ones. And so a high-quality essay on life safety
I tried charging regular duracells with low current. They recharged a little and worked a little more, but then ALL leaked.





Dumb and Dumber