Which batteries are better - alkaline or salt?
Many of us have heard at least once that batteries can be alkaline or salt. What does this mean and what type of batteries will last longer? Let's find out from this article.
The content of the article
What are salt batteries
Salt batteries refer to batteries that are of the “dry” type and contain a saline solution as an electrolyte. Not so long ago, this type of battery could be called a world leader in terms of quality and price, but over time, technology has stepped forward and the situation has changed.
Reference. Salt batteries were invented in 1865.
In a classic salt battery, you can find three main components: cathode, anode and electrolyte. The first two serve to generate current between themselves, and the third ensures the course of the reaction, which leads to the appearance of voltage. The cathode in such a battery is its shell, made of zinc, and manganese dioxide, thoroughly impregnated with zinc powder, is used as the anode.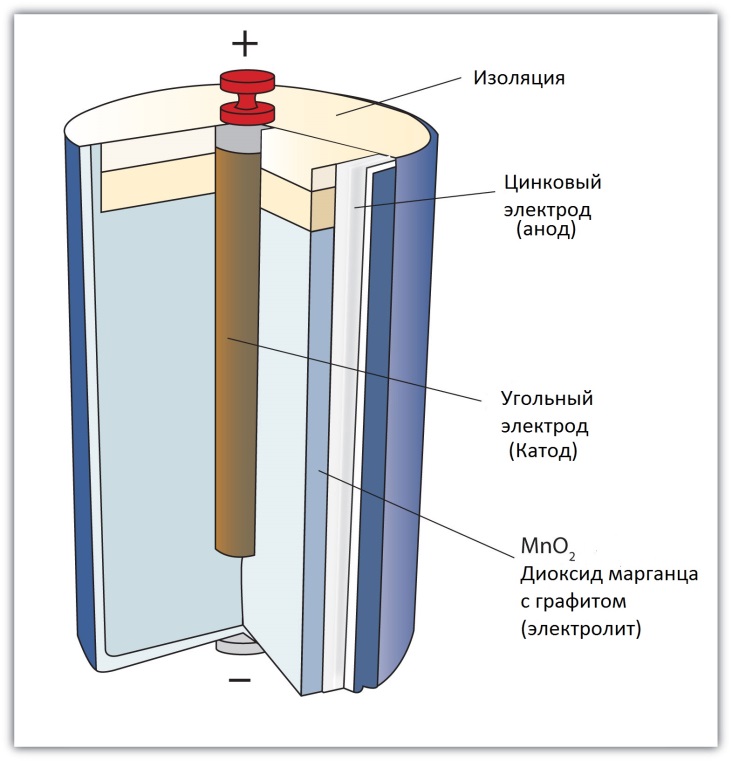
Reference. Due to the “impregnation” of the anode with zinc powder, salt batteries are sometimes called “carbon-zinc”.
The prototypes of modern batteries used a small amount of ammonium chloride thickened with starch as an electrolyte (now it can be found in some food products under the index E510).It was replaced with zinc chloride a little later, and some manufacturers even added the so-called calcium salt to zinc.
As we have already noted, in the process of a redox reaction, triggered by the interaction of three components, an electric current arises. This current is collected using the current collectors of the device, then directed to the electrodes and only then supplied to the consumer device.
Reference. The faster the redox reaction inside the battery occurs, the shorter the battery life.
Light weight and fairly low cost are the two main “trump cards” that salt batteries can boast of. By the way, if you don’t “force” them to work constantly, but let them “rest” from time to time, the service life of each individual element will increase significantly. If we are talking about a device that must work constantly, you can purchase two sets of batteries and change them one by one after a while.
“Salt” products are not without their disadvantages. These include a short shelf life (up to 3 years), self-discharge, instability of operation under critical temperature conditions, and sometimes oxidation of the shell and leakage. In addition, the average capacity of about 0.8 Ah cannot be called huge. However, for a remote control, a small flashlight or a radio, this is most often enough.
Features of alkaline batteries
Thomas Edison himself, as well as the talented American scientist Waldemar Jungner, had a hand in creating such a battery. At the beginning of the 20th century, these two bright minds designed the first device used for autonomous current transmission and containing alkali. By the way, “lye” in English sounds like “alkaline,” which makes it clear where “alkaline” gets its name.
It is worth noting that there is no big structural difference between alkaline (alkaline) and salt batteries, since the former also have electrodes and an electrolyte, thanks to which the reaction with the release of electric current is carried out. To prevent the device from exploding due to an uncontrolled reaction, the design includes a so-called safety membrane, as well as a gas chamber used to store gases generated during the discharge.
Reference. This type of battery has many advantages. These include a large capacity, long (up to 7 years) service life, inability to self-discharge, reliable sealing of the housing, high specific capacity, stable operation under constant vacuum conditions, and resistance to temperature changes.
Not without its shortcomings. Experts classify the large mass of alkaline batteries and their price as such. In addition, after they fail, they cannot be recharged using a special device and used again: a battery that has been ordered to live for a long time cannot be restored.
The largest manufacturers of alkaline batteries are Energizer, Duracell and Sony. The scope of application of their products is quite wide: from voice recorders, cameras and audio players to remote controls, powerful flashlights and alarms.
Which ones are better to choose?
The answer to this question is unlikely to be unambiguous, and when choosing batteries you need to proceed from a number of criteria. The main thing to do is to decide for what purpose the batteries will be used. If their scope of application is limited to the TV remote control, then there is no point in overpaying for branded alkaline batteries: although they will last longer, they are unlikely to pay for themselves. If we are talking about buying batteries, for example, for a camera, then in this case it is definitely better to give preference to “alkaline” ones, since they are not only more reliable in operation, but also discharge much more slowly.
When choosing a battery, you should also not forget about how a particular type copes with work in the cold. Thus, alkaline elements in 30-degree cold conditions will last on average twice as long.





