Battery operating principle
How do a wall clock, a TV remote control, or a radio-controlled children's toy work? Most people, without hesitation, will answer “from batteries” and, in principle, they will be right. But it is unlikely that any of them will be able to tell how exactly the portable battery is tripled, how it functions, and without which the entire process of transmitting electric current from the battery to the end consumer would be impossible. Let's fill this annoying gap in knowledge.
The content of the article
Battery operating principle
In order to understand the principle of operation of a conventional AA battery, you need to have a general understanding of its structure. So, any battery consists of three main elements - anode, cathode and electrolyte. Moreover, the latter can have virtually any state of aggregation: the cathode and anode placed in a saline solution, in principle, are also a “battery,” only in a form that is unusual for the average person.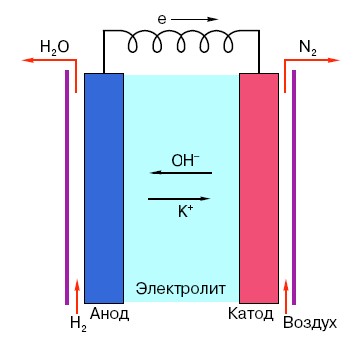
Interesting! The so-called “voltaic column”, invented by Alessandro Volta, also had all the elements necessary for the production of electric current. It consisted of zinc and copper plates stacked on top of each other, between which a cloth soaked in acid was placed as a “layer”.
The anode in such systems is the main source of electrons, which, as we know from a school physics course, have a negative charge.Negatively charged particles are attracted to positive ones, and in this case the cathode surface acts as a “plus”.
But this is not enough for an electric current to occur, because electrons also need a kind of “highway” - a medium that would support the interaction of the cathode and anode. It is here that an electrolyte appears “on stage” - a salt, alkali or acid capable of conducting current.
Let's look at the principle of operation using a specific example: there is a battery rated at 18 volts. The voltage between the electrodes in it is stable until it is connected to the network. As soon as a consumer appears (for example, an ordinary light bulb), the voltage begins to gradually decrease, current begins to flow from the “negative” electrode to the “positive” one, and a chemical reaction occurs in the electrolyte aimed at maintaining the potential difference between the electrodes.
Reference. The more energy the consumer requires, the more intense the reaction inside the battery and the faster it will fail.
How a rechargeable battery works, how it differs from a regular one
So, we have looked at the classic “finger” and “little finger” batteries and we know that the service life of most of them is strictly limited (no matter what famous manufacturers say). But what about the so-called batteries - battery-type batteries that can not only consume energy during the reaction process, but also accumulate it and store it for a long time?
In order to understand the principle of operation of the battery, it is necessary to turn to chemistry. Let's take as an example... A regular charcoal fire.No matter how beautiful and fascinating the flame looks, any chemist observing it knows that this process is just a long-term reaction of fuel oxidation. Burning coal interacts with oxygen and as a result of this reaction we get:
- carbon dioxide;
- light;
- warm.
And if the last two points are capable of warming the soul and body, then we cannot use carbon dioxide in any way, because it is a by-product of the reaction, which is, in fact, its waste. The oxidation reaction stops when the starting elements: oxygen and coal run out. Stopping the reaction in a battery occurs in exactly the same way when the starting substances are completely exhausted and only “waste” remains.
In the battery, everything happens a little differently. The fact is that the reaction occurring in it belongs to the category of reversible, that is, under certain conditions it can be “reversed”, returning all substances to their original state. It is the possibility of a reversible reaction occurring in the battery that allows it to be charged.
In a battery connected to the network, the reaction proceeds in the opposite direction, and the current flows from “plus” to “minus”, and not vice versa. As a result, the reaction product forms the starting substances, and the owner of the battery receives available “recovered” energy in a portable format. That's all!





