What does a battery consist of and how does it work?
Batteries are widely used in daily life. They make it a little easier. For example, you don’t need to spend a lot of effort to turn on the TV using the remote control or use a calculator. Thanks to them, you always know the time or can use a wireless computer mouse. However, not everyone knows what types of batteries there are, and most importantly, what is inside them.
The content of the article
How different types of batteries work and what's inside them
The very first prototypes of batteries appeared more than 2 thousand years ago in Mesopotamia. Yes, this is not a joke. Such a battery consisted of a clay vase and a copper rod, which was filled with a special composition resembling bitumen. This composition could also be replaced with wine vinegar. As a result, it was possible to obtain a voltage of about 0.5-1 V. Such a device was called the “Baghdad battery” (because of the place where it was found). Today this artifact is kept in the National Museum of Iraq.
However, these were the very first batteries, the design of which can be called primitive. Today, their production and composition have changed dramatically when compared with their “ancestors”.
The design of modern storage elements is simple. The differences between each type are minimal. At the heart of any design there is a positive pole (anode) and a negative pole (cathode). It also contains an electrolyte.The main characteristics and parameters of the battery depend on it:
- energy intensity;
- voltage;
- life time;
- performance at subzero temperatures.
In addition to the main elements, any battery has an insert that acts as a gasket, a diaphragm, a case and a carbon rod.
In general, the “filling” itself can be lithium, alkaline and salt. The last option, one might say, has long outlived its usefulness. The fact is that salt batteries have more disadvantages than advantages (when compared with other batteries). But what does the electrolyte look like?
There are several chemicals inside the housing. In salt batteries, these are zinc, magnesium dioxide, potassium hydroxide, and passive carbon.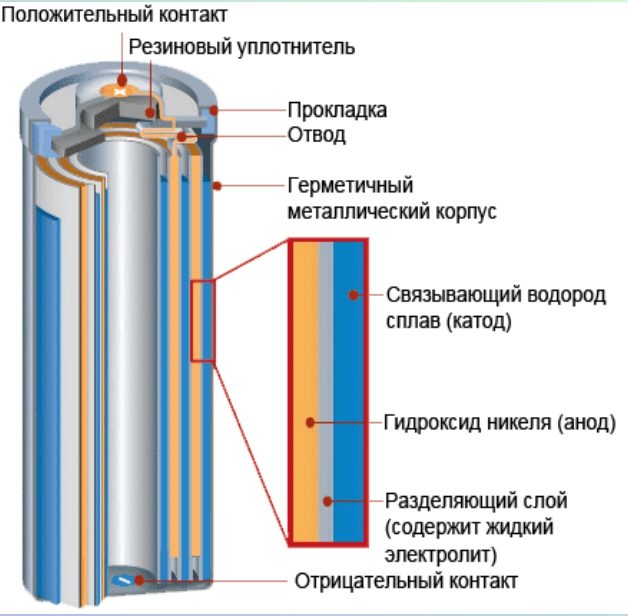
Reference. When batteries are not in use, they can restore charge or, in other words, smooth out local irregularities that are caused by cell discharge. This process allows you to slightly extend the service life of the product.
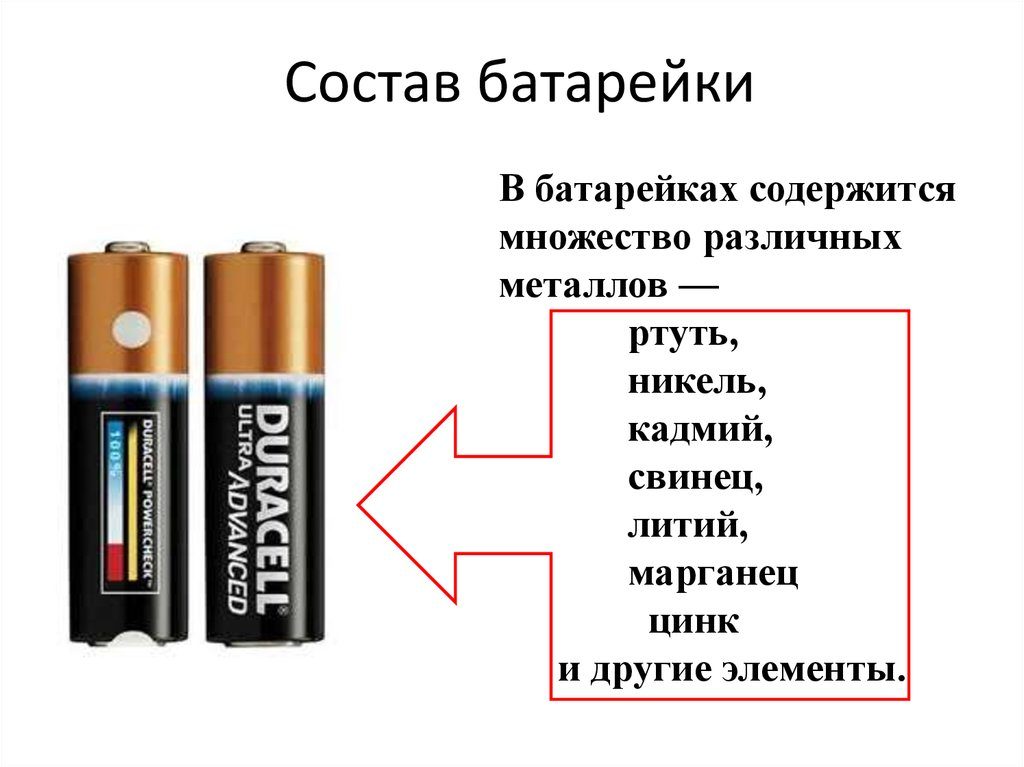
In addition to the above elements, the battery may also include others:
- nickel;
- lithium;
- lead;
- mercury - today it is practically not used;
- iron;
- aluminum;
- lead;
- manganese.
In alkaline batteries, alkali acts as the electrolyte. Thanks to its properties, this product operates stably throughout its entire service life, discharges more slowly, operates even at sub-zero temperatures and has a longer service life.
Inexperienced users may think that the chemical reactions undergone are distinguished by their complexity. In fact, this is not so - everything is quite simple here. A chemical reaction moves negatively charged particles (electrons), which ultimately creates an electric current.
Lithium batteries contain (as the name suggests) lithium. It acts as a cathode and has the highest negative potential. It is worth noting that this chemical element is organic, due to which the battery has improved characteristics.
If we look at the types, then each battery can have its own elements. For example, a “tablet” contains mercury oxide and zinc powder. The crown has a more complex composition:
- two positive and negative contacts each;
- plastic plates that are located at the top and bottom of the product;
- six separate batteries connected to each other;
- coal rod;
- insulation plate;
- wrapper.
This product also includes a housing, but we will talk about it separately.
What is the battery case made of?
Any battery has a durable case that protects the element and performs a number of important functions. Various components of the future battery are placed in it: manganese dioxide, thickeners, barium sulfate, catalyst, zinc, etc.
However, not everyone knows that it is made from various metals. For many it is simply metal. In fact, no - everything is much more complicated.
First of all, it can be made from zinc. More often they are equipped with salt batteries. Recently, there have been cases made of iron or special stainless steel. This case is characterized by increased strength and level of protection.
It consists of an upper and lower plate, on which it is marked where is “minus” and where is “plus”. The body also includes a side plate. It is its main part. In fact, the production itself begins with the cutting of such plates on a machine.After which they are twisted into a tube, which is called the body.
Reference. The body can be made not in the form of a tube, but in the shape of a square. Batteries of this structure have the highest voltage - up to 9 Volts.
Despite the high voltage, this type of battery is not popular and is not particularly in demand among domestic consumers. The reasons lie on the surface, and in the literal sense of the word. Firstly, such a product is distinguished by a thick shell that protects chemical elements from external adverse influences and falls. Secondly, only a small part of the devices are designed for this form of battery, that is, the batteries simply will not fit in them and will not fit into the connector.





