What are salt batteries
Today you won’t surprise anyone with a miniature radio receiver, and flashlights have generally acquired a bunch of all kinds of modifications, various echo sounders and many other electronic gadgets... All this does not work from the network, but nevertheless requires electrical power. In order for these devices to have current, they use galvanic cells, or, in simple terms, batteries.
They were first learned about back in 1800, they were invented by the Italian Alessandro Volt. Batteries come in a variety of shapes and sizes, and they have different voltages and wattages. They use different elements for power supply. The most common are alkaline and salt batteries.
The content of the article
Salt battery - what does it mean?
Salt batteries are small sources of electric current that are created inside a simple device as a result of a chemical reaction occurring. You can also find another name for them - carbon zinc or carbon. This is a very cheap device, but its energy potential is very low. They are used only in devices that consume little electrical current. For other devices that are more demanding of electricity, they are of little use, since their supply will quickly run out.
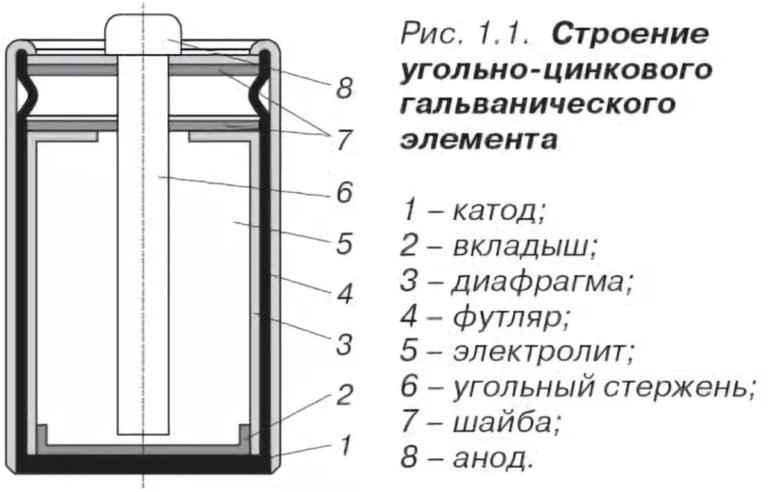
Composition and design of salt batteries
The salt battery is very simple. It includes the following elements:
- The cathode is the housing itself, which is made of zinc, has good corrosion resistance and is well cleaned.
- For the anode, a pressed agglomerate is used, which is impregnated with electrolyte.
- Ammonium chloride or zinc chloride is used as an electrolyte in the battery, to which starch is added to thicken.
- In the very center there is a current conductor made of coal and impregnated with a paraffin composition.
- At the top there is a gas chamber. Gases formed during a chemical reaction accumulate in it.
- There is a gasket at the top for sealing.
- The battery is equipped with a protective case made of cardboard or tin. Its task is to protect against corrosion and electrolyte leakage.
Types and sizes
There are many batteries of this type. Each size has its own marking. At the direction of the International Electrotechnical Commission, a set of letters and numbers began to be used to identify them. And sometimes they are identified according to ANSI/NEDA or GOST/TU standards.
Among all the variety, there are two sizes of batteries that are recognized as standard. They are easily distinguishable externally. The most common are the AA battery, also called the “finger” battery, as well as the AAA or “pinky” battery. Their voltage is the same, and the body is cylindrical in shape.
In addition to these, there are three other types of batteries. One of them looks like a small barrel and is marked C or LR 14.
At one time, they began serial production of batteries for flashlights marked D or LR 20, which looked like a large barrel. Despite the fact that they were made for flashlights, they also worked great in portable radios.
The Soviet Union produced batteries labeled R10.They were used to operate measuring instruments, as well as in toys.
Cylindrical batteries, with their positive pole located at the end, have a protrusion. Their other side is completely flat.
But element 6 F22, which is also called crown, is produced in the shape of a rectangle. It somewhat resembles a matchbox. The battery size is small. The crown's plus and minus are located on one end.
Positive and negative properties of salt batteries
Any thing in our life has its positive and negative sides. This is how this world works. The salt battery is no different from the others. One of its positive qualities is that it is small in size and does not weigh much. It is very comfortable. And if you sometimes let it lie idle, then it can last a little longer.
You can invigorate a “tired” battery by vigorously shaking it or hitting it with your hand. This will smooth out the sticky lumps of electrolyte inside it and it will continue to function successfully for some time.
But they have more negative properties:
- are not stored for long (usually their shelf life does not exceed three years);
- have a tendency to lose charge on their own;
- the electrolyte often dries out;
- do not tolerate temperature changes well;
- from long-term storage, their body oxidizes and the electrolyte leaks out - therefore, it is recommended to remove them from the device if it is not in use;
- they have a small energy capacity.
Where are salt batteries used?
Since this type of battery has very modest energy indicators, their main purpose is to provide electricity to devices with low consumption. These are small radios, flashlights, remote controls, and testers. Such batteries are produced at various factories. Among the domestic ones, the most famous are “Cosmos”, “Energy” and “Photon”, and they are produced abroad by Sanyo and GP.
Such a battery costs little, and besides, it is very light. But you won’t be able to stock up on them for future use: after lying around for at least three years, they will break down, and you don’t even need to use them, because they discharge themselves.
Since the capacity of this battery is low, it can only be used in devices that have low electricity consumption.
Externally, rechargeable and non-chargeable batteries do not differ, however, this can be determined. If the battery is rechargeable or, in other words, it is a battery, then there should be a mark on the case about the capacity. If there is no such marking, then this means that we have a simple battery.
Many “traditional craftsmen” say that they can also be charged, but this is more of a fantasy: no matter how long you keep it on charge, there will be no positive effect. You can only suffer from an overheated case or leaking electrolyte. It is better to buy alkaline batteries or normal rechargeable batteries.
Reference. The simplest salt battery can be made with your own hands, it’s very simple.
All you need for this:
- several coins of 50 kopecks;
- foil;
- paper;
- saline solution
Coins must first be cleaned in a vinegar solution. This will remove all plaque and dirt.
The assembly technology is as follows: take a coin, soak the paper in a saline solution, then take the foil. By repeating this action several times, we get a small column.The coin located at the very top is the positive pole, and the foil located at the bottom is the negative pole.
Since there is a different potential between the coin and the foil, which is created by the electrolyte (in our case, a saline solution), an electric current arises. In fact, we repeated Volta's invention and assembled a Voltaic pillar. The more coins we use, the more voltage we get. However, old coins will no longer be suitable for repeating the experiment, as they will develop a coating of rust.