DIY battery: from lemon, coins, potatoes, jars
Perhaps for some this will be a discovery as significant as the discovery of America by Columbus, that there is electricity everywhere around us. It literally permeates our entire lives. But even knowing this sometimes does not prevent our eyes from widening when we learn that tension can be obtained from the most ordinary things and even from food. Using what you have in the kitchen or garage, it is quite possible to build a simple battery at home. 
The content of the article
lemon battery
Even from this fruit you can get electricity. To do this you need to prepare the following things:
- one lemon;
- a piece of something steel;
- something made of copper;
- and two pieces of wire for insulation.
First we will need to clean up our steel and copper items. Regular sandpaper will help with this.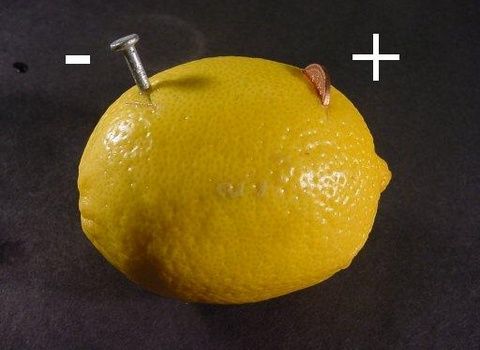
Reference. An object made of steel can be the most ordinary nails. There are plenty of them in any garage. And for “something made of copper”, you can use coins in denominations of ten and fifty kopecks.
Now we stick a nail and a coin into the lemon. Between them you need to make a gap of about three centimeters. These will be our electrodes, all that remains is to connect the wires to them. You can just stick it right next to it. The coin is our positive contact, and the nail, therefore, is negative. 
Reference. Lemon can be successfully replaced with an ordinary apple.The main thing is to choose the most sour one that you don’t mind using for experiments. And the acid is useful for the reaction to proceed.
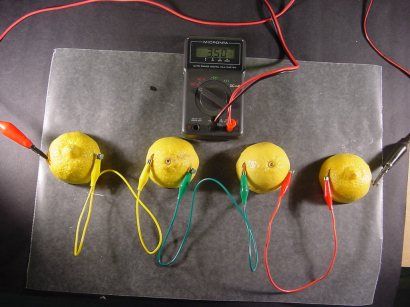
A lemon or apple battery (if you take only one fruit) will produce about 0.5 or 0.7 volts. This is very little - you can’t charge even the simplest mobile phone. You need to somehow bring the voltage to three or even five volts. But how? Yes, it’s very simple - connect more fruits into a single chain.
Reference. To increase the charge of our circuit, it can be charged. It is enough to include a crown battery or even a mobile phone charger into the circuit.

Making lemons or apples produce electricity becomes possible because the copper element interacts with the steel element. The acid contained inside the fruit triggers this reaction. As long as there is at least a drop of acid inside or as long as the contacts are intact, the battery will continue to work.
Electricity in a bank
Even from an ordinary can you can build something similar to the very first battery in the world. To do this you will need:
- a simple glass jar (you can use a glass);
- zinc or aluminum plate;
- strip of copper;
- several wires;
- ammonia, also known as ammonium chloride;
- tap water.
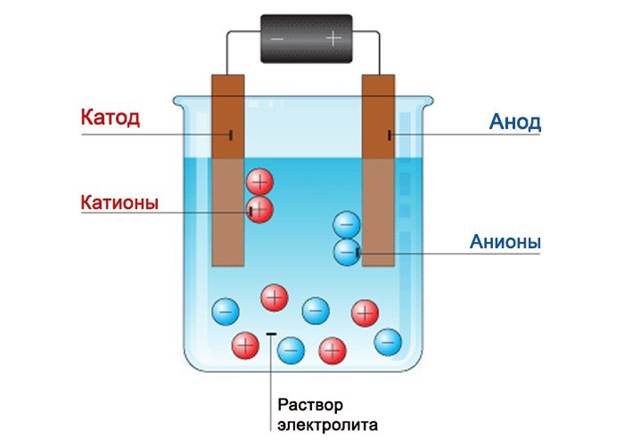
Our battery will have an aluminum plate as the anode, and a copper plate will serve as the cathode. Their size must be selected so that their area is equal to the palm of a person. This will make our battery more efficient. Solder the wires to the plates. Now our task is to install the plates in the jar so that they do not touch each other. And the height of these plates should be greater than the can itself.
It's electrolyte time. It's easy to do. Mix ammonia with water. For every 0.1 liter of water you need to add 50 grams of powder.Mix everything thoroughly and pour into a jar. Instead of ammonia, you can also use sulfuric acid. To do this, it will need to be brought to a twenty percent state.
Important! If you make an electrolyte based on sulfuric acid, then when diluting it you need to pour the acid into water, but not vice versa. Otherwise, the water can simply boil, and as a result of a violent reaction, everything will splash out. In addition, do not forget that when working with acid you need to wear protective equipment.
Fill the jar with the resulting solution. If you combine several cans into a single circuit, you will get a very good battery, the energy of which is quite enough to charge a fairly powerful device. This battery is similar to salt batteries.
DIY coin battery
Even the coins that are in your wallet or piggy bank can generate electric current. From coins you can build the simplest galvanic cell, which in science is called a Voltaic column. We need to prepare:
- several copper coins (such as coins of fifty and ten kopecks);
- food foil;
- several sheets of paper;
- table vinegar or a solution of water and salt.

Now let's assemble our energy column:
- We take a piece of paper, soak it in vinegar and attach it to a coin.
- Place foil on paper.
- Now again the coin.
- Until we finish adding the coins, we repeat everything sequentially.
- As a result, there will be a coin at one end of the structure. This is the positive pole, the other end will have foil. This is the negative pole.
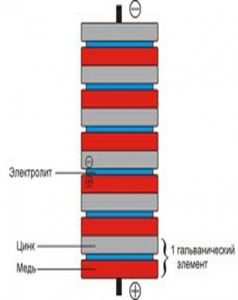
The more coins you can collect, the greater the tension. The coins cannot be reused. After the experiment they will already be rusty.
Electricity in a beer can
After drinking canned beer, do not rush to throw away the empty can. It will make a good battery. To do this you will need:
- beer can (they are made from food grade aluminum);
- fire charcoal or coal dust;
- paraffin candle;
- graphite pencil lead;
- water and salt;
- a piece of foam plastic - the foam should be more than a centimeter thick.
Cut off the top of the can. We cut a circle out of foam plastic so that its diameter matches the jar. We make a hole in the foam, but not through. We will install a graphite rod into the hole. Place polystyrene foam on the bottom of the jar and insert the rod. The graphite rod should be exactly in the center of the can. We fill everything around the rod with coal dust.
Important! Make sure that the rod does not touch the walls of the jar.
Now we make a solution of salt and water. To do this, take half a liter of water and add three tablespoons of salt into it. Mix everything thoroughly so that all the salt is completely dissolved. It will dissolve faster and better if the water is heated. We pour our electrolyte into a jar and seal everything with paraffin. The graphite rod should rise above the level of the can.
We connect one wire to the rod - this is the positive contact. And the second wire to the wall of the can is the negative contact.If you make a circuit of two cans, you can get a voltage of three volts. This battery can power a light bulb.
Potato battery
If you have potatoes at home, then this is quite an energetic thing. True, one-time use. The potato battery can only be used once. For example, on a hike.
To obtain the battery, we will prepare the following elements:
- you will need large potatoes;
- copper wires in insulation;
- toothpaste;
- wood chips or toothpicks;
- table salt.
Cut the potatoes into two parts. It is advisable to do this lengthwise to get a larger cutting area. Cut out the core in one half to create a hole. Place a mixture of toothpaste and salt in this hole. The composition should fill the entire recess. This mixture will act as an electrolyte.
We make two holes in another piece of potato. The distance between the holes should be such that both are located above the electrolyte mixture when both halves are connected. These holes are needed for wires. The ends of the wires must be stripped of insulation to a length of two centimeters. Now we connect both parts of the potatoes and, so that they do not fall apart, we fix them with toothpicks.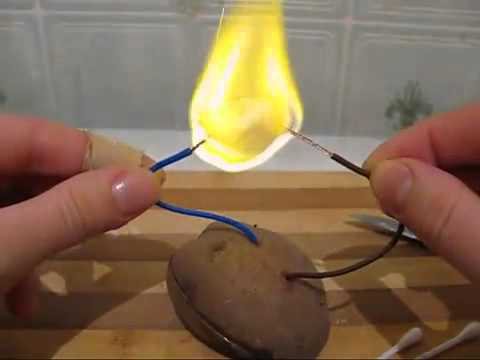
We wait five minutes for the reaction to begin. Now we close the wires and see a spark at the end. This is how you can safely light a fire with a potato battery at a camping stop.
Conclusion
Naturally, all the battery options considered, although they work, cannot fully replace either a battery or an accumulator. But who is stopping you from doing such experiments in order to better understand the structure of these physical elements and chemical processes?





